Ozone Disinfection / Ozone Contact Time Kinetics
Water is disinfected but never completely sterilized in the water treatment process. This disinfection is a two part process that includes:
-
Removal of particulate matter by filtration. A rule of thumb is that high turbidity in the effluent is a potential health risk, because viruses and bacteria can hide within the rough texture of particulates. Therefore, removal of the particulates reduces the chance of pathogenic microorganisms in the effluent.
-
Inactivation of pathogenic microorganisms by chlorine, chlorine dioxide, ozone, or other disinfectants
Contact time and kinetics are simply a measure of the inactivation due to time and concentration of the disinfectant. The USEPA has developed regulations for the minimum kill percentages (inactivation) necessary for public water to be considered potable. These regulations include a minimum disinfection of:
- 3 log (99.9%) for Giardia lamblia cysts
- 4 log (99.99%) for enteric viruses
In "water treatment terms" 1 log inactivation is referred to as 1 credit inactivation. Different types of filtration are assigned certain removal credits. For example, conventional filtration is worth 2.5 credits for Giardia cysts. Since the EPA requires 3 log (credit) removal, an additional 0.5 credit inactivation from disinfection must be attained.
Varying degrees of disinfection can be attained by altering the type and concentration of disinfectant, as well as the time water is in contact with the disinfectant. The decision to use one type of disinfectant versus another will set the precedence for the remainder of the values needed to attain the proper disinfection. The time untreated water is exposed to the disinfectant and the concentration of that disinfectant are the main factors in the equation that will be discussed in the next section. (Notice that the units of contact time are (mg/l)(min).)
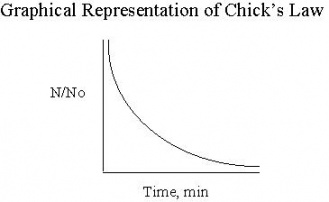
A relationship between kill efficiency and contact time, was developed by Harriet Chick while she was a Fellow in the Pasteur institute in Paris, France. The research yielded data supporting her relationship that is shown in Figure 2 below. (No) represents the initial number of organisms and N is the number of organisms at time t. As contact time between water and disinfectant increases, the ratio of No/N decreases as Chick's Law predicts.
Watson later modified Chick's equation to account for varying types of disinfectants. He developed coefficients that better represented the strength of the disinfectant as well as the pH of the water. From this research, the coefficient of specific lethality (lambda) was developed. Watson’s modification of Chick’s equation is shown below.
Factors Affecting C*t Values
-
As pH increases the value of C*t also needs to be increased. This can be explained by examining the effects of pH on free chlorine. As the pH increases, more of the weak disinfectant (OCl-) exists than the strong disinfectant (HOCl-), thus increasing the C*t value. Refer to Table below.
-
The greater log removal needed, the greater the C*t needs to be, as can be seen in Table.
| Log Removal |
pH < 6 |
pH 6.5 |
pH 7.0 |
pH 7.5 |
| 1.0 |
46 |
54 |
65 |
79 |
| 1.5 |
69 |
82 |
98 |
119 |
| 2.0 |
91 |
109 |
130 |
158 |
| 2.5 |
114 |
136 |
163 |
198 |
C*t for Removal of Giardia Cysts in Relation to Log Removal and pH
Information from the Virginia Department of Health Waterworks Regulations
-
The strength of a disinfectant directly affects the C*t. For a weak disinfectant, the C*t will have to be higher than for a strong disinfectant. As Table 2 below shows, ozone is the strongest disinfectant, thus the C*t value required is less when compared to chlorine and chlorine dioxide.
-
Different organisms have different resistances to disinfectants. If an organism has a strong resistance to a certain disinfectant, the C*t will be higher than for an organism with a weaker resistance. Refer to Table below.
-
Different organisms have different resistances to disinfectants. If an organism has a strong resistance to a certain disinfectant, the C*t will be higher than for an organism with a weaker resistance. Refer to Table below.
| Organism |
Free Chlorine (pH 6-7) |
Chlorine Dioxide (pH 6-7) |
Ozone (pH 6-7) |
| E.Coli |
0.034-0.05 |
0.4-0.75 |
0.02 |
| Rotavirus |
0.01-0.05 |
0.2-2.1 |
0.006-0.06 |
| Giardia lamblia cysts |
47-150 |
- |
0.5-0.6 |
| Crytosporidium parvum |
7200* |
79* |
5-10* |
C*t Values for the 99% Inactivation at 5 Degrees Celsius of Organisms Using Various Disinfectants
* 99% inactivation at 25 degrees C

